

Powering Progress: Real Regulatory Wins, Transforming Compliance into Success!
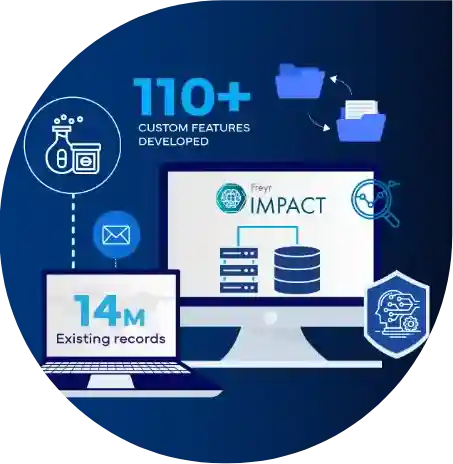
Join us on a remarkable journey as Freyr transforms the Regulatory Intelligence ecosystem for a top 5 global bio-pharmaceutical giant. Faced with fragmented Regulatory and decision-making processes, our custom solution integrated advanced technologies to centralize their records and streamline global compliance tracking, truly transforming their Regulatory intelligence processes.

The customer’s portfolio comprised of two CAD software solutions, designed for evaluating chest radiography and mammography images. These SaMDs, approved by the MFDS, Korea, and CE-marked in compliance with the EU MDD, represented moderate-risk technologies. With ambitious plans for expansion, the customer was poised for aggressive growth. Freyr played a pivotal role, providing tailored Regulatory strategies and market entry solutions to accelerate their global expansion across multiple countries spanning ASEAN, MENA, and LATAM regions.
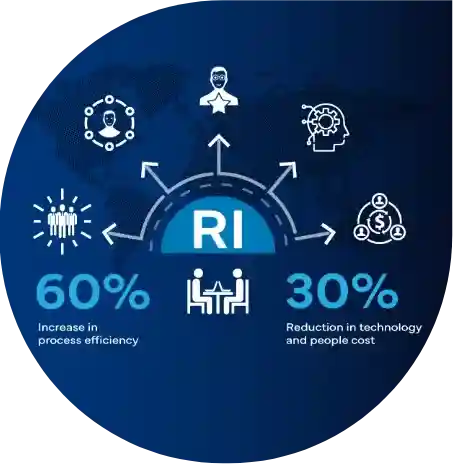
Uncover the story of a prominent EU pharmaceutical innovator grappling with Regulatory intelligence hurdles. Drowning in data from multiple tools, they struggled with inefficient processes and reactive decision-making. Freyr's tailored 360-degree consulting approach came in as a complete game-changer, significantly boosting efficiency and reducing operational costs.

A leading US pharmaceutical company had faced significant challenges in managing over 15 new and approved products (ANDAs/NDAs) due to a lack of in-house Regulatory expertise and limited FDA knowledge. Seeking comprehensive Regulatory support, they had turned to Freyr. Freyr's expert team provided strategic guidance, submission support, and ongoing Regulatory operations management, offering end-to-end Regulatory solutions that ensured seamless submission processes and continuous Regulatory compliance. As a result of Freyr's support, the pharmaceutical company achieved zero rejections and maintained Regulatory excellence.
 their SaMD radiology solution" width="453" height="464" />
their SaMD radiology solution" width="453" height="464" />
The customer required Freyr to migrate content from the legacy application (FrameMaker) to Vasont Inspire Component Content Management System (CCMS). Freyr tailored Technical Writing solutions enabled the customer to achieve their goal with a 40% quicker turn-around-time.

A US-based pharmaceutical company had faced high attrition rates and limited in-house expertise, struggling with the Regulatory operations of 70+ ANDAs/NDAs. They had turned to Freyr for comprehensive Regulatory services. Freyr had provided strategic guidance and submission management, transforming the company's Regulatory operations. This support ensured seamless operations, timely approvals, and enhanced compliance.